Pfizer on Friday said it would stop developing the twice-daily version of its experimental weight loss pill after obese patients taking the drug lost significant weight but had trouble tolerating the drug in a mid-stage clinical study.
The drugmaker observed high rates of adverse side effects, which were mostly mild and gastrointestinal, among patients. A significant share of patients also stopped taking the drug.
“At this time, twice-daily danuglipron formulation will not advance into Phase 3 studies,” the company said.
But Pfizer said it still plans to release phase two trial data on a once-a-day version of the drug in the first half of 2024, which will “inform a path forward.” The pharmaceutical giant will wait to see that data before deciding whether to start a phase three study on the once-daily pill, which Wall Street views as the more competitive form of the treatment.
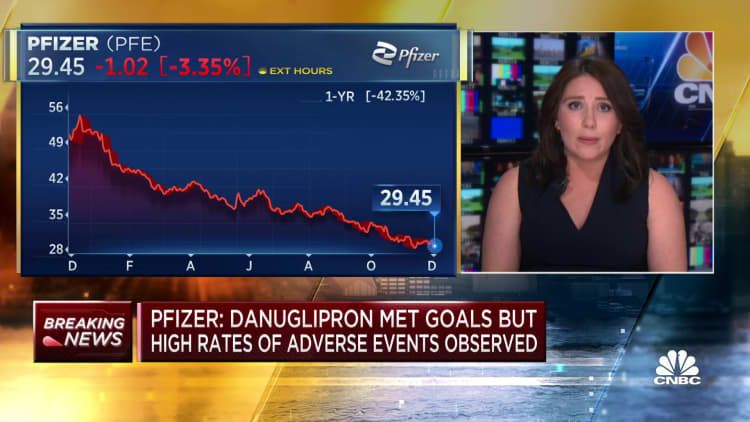
Shares of Pfizer fell 4% in premarket trading Friday after it announced the trial results.
Still, the data on the twice-daily drug is a blow to Pfizer’s hopes to win a $10 billion slice of the booming weight loss drug market, which CEO Albert Bourla has said could grow to $90 billion. The company is betting on a successful weight loss pill to help it rebound from plummeting demand for its Covid products and a roughly 40% share price drop this year.
But investors have been pessimistic about Pfizer’s potential in the weight loss drug space since the company scrapped a different once-daily pill in June and proceeded with the less attractive danuglipron. Now, Friday’s data puts Pfizer even further behind the dominant players in the weight loss drug market, Eli Lilly and Novo Nordisk, which are racing to develop more convenient pill versions of their blockbuster weight loss and diabetes injections.
Pfizer’s phase two trial on its twice-daily pill followed around 600 obese adults who did not have Type 2 diabetes. The trial examined the drug’s effect on weight loss after 26 or 32 weeks, at different dosage amounts ranging from 40 milligrams to 200 milligrams.
Like Novo Nordisk’s Wegovy and Ozempic, Pfizer’s pill works by mimicking a hormone produced in the gut called GLP-1, which signals to the brain when a person is full.
Pfizer said the trial on danuglipron met the primary goal of demonstrating “statistically significant” reductions in body weight.
Patients who took the pill twice a day lost 6.9% to 11.7% of their body weight on average at 32 weeks, and from 4.8% to 9.4% at 26 weeks.
Meanwhile, patients on a placebo gained 1.4% of their body weight at 32 weeks and 0.17% at 26 weeks.
When adjusting for the difference between the weight gain observed in patients who took the placebo, Pfizer’s twice-daily pill caused 8% to 13% weight loss on average at 32 weeks and 5% to 9.5% at 26 weeks.
The company said high rates of adverse events were observed among patients in the study, with up to 73% experiencing nausea, up to 47% vomiting and up to 25% experiencing diarrhea. More than 50% of patients across all dose sizes stopped taking the pill, compared to roughly 40% among those on the placebo, according to Pfizer.
No new safety issues were observed, and danuglipron was not associated with increased liver enzymes like Pfizer’s other discontinued weight loss pill.
Data from the phase two trial will be presented at a future scientific conference or published in a peer-reviewed journal.
Wall Street’s expectations
The tolerability issues align with some analysts’ predictions ahead of the data release.
Leerink Partners analyst David Risinger wrote in a Monday note that the proportion of patients who discontinue treatment with Pfizer’s twice-daily danuglipron in the phase two trial would likely be higher than those who stopped taking a once-daily pill from Eli Lilly.
By comparison, 10% to 21% of patients who took Eli Lilly’s pill, orforglipron, in a mid-stage trial discontinued the treatment at 32 weeks due to adverse side effects, he noted.
Risinger said that’s likely because danuglipron’s total daily dose is far higher, which may cause more adverse effects. Patients on the highest dose size of Pfizer’s pill took 400 milligrams each day, while those on the highest dosage of Eli Lilly’s drug took 45 milligrams a day.
Pfizer’s phase-two trial also didn’t allow downtitration, or decreasing the dose of a drug over time once a specific response has been achieved. Eli Lilly’s mid-stage trial on its pill did.
There is hope that patients will better tolerate the once-daily version of danuglipron compared to the twice-daily form. Pfizer appears to believe a once-daily version of the drug could lessen gastrointestinal side effects, according to some analysts.
They pointed to Pfizer’s second-quarter earnings call, when the company’s chief scientific officer, Mikael Dolsten, suggested that a once-daily version may improve a patient’s tolerability of the drug, which could lessen the gastrointestinal side effects “that have been seen as limiting” danuglipron.
But the effects will be unclear until the mid-stage trial data is released next year.
Notably, the weight loss caused by twice-daily danuglipron appeared to fall short of analysts’ expectations.
Ahead of the data release, several analysts said Pfizer’s twice-daily pill has to be about as effective as Eli Lilly’s once-a-day pill to be competitive. That means at least a 14% to 15% weight loss, Cantor Fitzgerald analyst Louise Chen told CNBC earlier this month.
Risinger also wrote in October that Pfizer’s danuglipron needs to show weight reduction in the “mid-teens” percentages to be considered competitive with Eli Lilly’s pill.
Obese or overweight patients who took 45 milligrams of Eli Lilly’s pill once a day lost up to 14.7% of their body weight, or 34 pounds, after 36 weeks, according to the company’s phase-two trial results.
Eli Lilly’s results appear consistent with the weight reduction caused by a high-dose oral version of Novo Nordisk’s semaglutide – the active ingredient used in the diabetes drug Ozempic and weight loss treatment Wegovy – but came over a shorter trial period.
More than 2 in 5 adults have obesity, according to the National Institutes of Health. About 1 in 11 adults have severe obesity.
Clarification: This story was updated to reflect that some weight-loss data was adjusted to include results from the placebo group.
Credit: Source link













